Introduction
Acute pancreatitis refers to inflammation of the pancreas. Its incidence is increasing, with around 30 per 100,000 cases each year in the UK at present. Mortality figures can range between 5-30%, depending on severity.
It can be distinguished from chronic pancreatitis by its limited damage to the secretory function of the gland, with no gross structural damage developing. Repeated episodes of acute pancreatitis can eventually lead to chronic pancreatitis.
Aetiology
The majority of acute pancreatitis cases occur secondary to gallstone disease or excess alcohol consumption. However, causes are wide ranging and a popular mnemonic is ‘GET SMASHED’:
- Gallstones
- Ethanol (Alcohol)
- Trauma
- Steroids
- Mumps
- Autoimmune disease, such as Systemic Lupus Erythematosus (SLE) or Sjogren’s syndrome
- Scorpion venom (a rare and unlikely cause in most countries)
- Hypercalcaemia
- Endoscopic retrograde cholangio-pancreatography (ERCP)
- Drugs, such as Azathioprine, NSAIDs, or Diuretics
Unfortunately, no evident cause will be found in 10-20% of patients with acute pancreatitis
Pathophysiology
Each cause described above will trigger a premature and exaggerated activation of the digestive enzymes within the pancreas. The resulting pancreatic inflammatory response causes an increase in vascular permeability and subsequent fluid shifts (often termed “third spacing”).
Enzymes are released from the pancreas into the systemic circulation, causing autodigestion of fats (resulting in a ‘fat necrosis’) and blood vessels (sometimes leading to haemorrhage in the retroperitoneal space). Fat necrosis can cause the release of free fatty acids, reacting with serum calcium to form chalky deposits in fatty tissue, resulting in hypocalcaemia.
Severe end-stage pancreatitis will eventually result in partial or complete necrosis of the pancreas.
Clinical Features
Patients will classically present with a sudden onset of severe epigastric pain, which can radiate through to the back, with nausea and vomiting.
On examination, there is often epigastric tenderness, with or without guarding. In severe cases, there may be haemodynamically instability, due to the inflammatory response occurring.
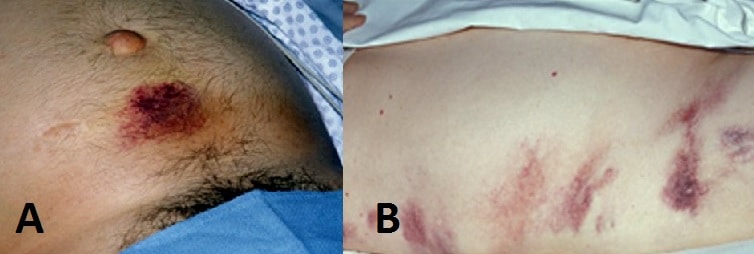
Less common signs that are often described are Cullen’s sign (bruising around the umbilicus, FIg. 2A) and Grey Turner’s sign (bruising in the flanks, Fig. 2B) , representing retroperitoneal haemorrhage. Tetany may occur from hypocalcaemia (secondary to fat necrosis) and, in select cases, gallstone aetiology may also cause a concurrent obstructive jaundice.
Differential Diagnosis
There are a wide variety of causes of an acutely painful abdomen, as discussed elsewhere. However causes specifically resulting in abdominal pain that radiates through to the back include abdominal aortic aneurysm, renal calculi, chronic pancreatitis, aortic dissection, or peptic ulcer disease.
Investigations
Laboratory Tests
Routine blood tests, as per investigation of any acute abdomen, are required. Specifically for acute pancreatitis, it is important to consider:
- Serum amylase or serum lipase – diagnostic of acute pancreatitis if 3x the upper limit of normal*
- Amylase can also be marginally raised in pathologies such as bowel perforation, ectopic pregnancy, or diabetic ketoacidosis; raised serum lipase is more accurate for acute pancreatitis, as it remains elevated longer than amylase
- LFTs – assess for any concurrent cholestatic element to the clinical picture
- Patients with acute pancreatitis noted that an alanine transaminase (ALT) level >150U/L has a positive predictive value of 85% for gallstones as the underlying cause
*Serum amylase or lipase levels do not directly correlate with disease severity
Risk Scoring
The modified Glasgow criteria is used to assess the severity of acute pancreatitis within the first 48 hours of admission. Any patient scoring with ≥3 positive factors within the first 48hrs should be considered to have severe pancreatitis and a high-dependency care referral is warranted.
Helpfully, the mneumonic to remember the score is PANCREAS: pO2 <8kPa, Age >55yrs, Neutrophils (/WCC) >15×109/L, Calcium <2mmol/L, Renal function (Urea) >16mmol/L, Enzymes LDH>600U/L or AST>200U/L, Albumin <32g/L, Sugar (blood glucose) >10mmol/L
Other risk stratification scores that can be used scoring severity of acute pancreatitis include the APACHE II score, the Ranson Criteria, and Balthazar score (CT scoring system).
Imaging
An abdominal ultrasound scan may be requested if the underlying cause is unknown; it is typically used to identify any gallstones (as a potential underlying cause) and any evidence of duct dilatation
Whilst not routinely performed for acute pancreatitis, an AXR can show a ‘sentinal loop sign’. This is a dilated proximal bowel loop adjacent to the pancreas, which occurs secondary to localised inflammation. A CXR should be undertaken to look for pleural effusion or signs of ARDS.
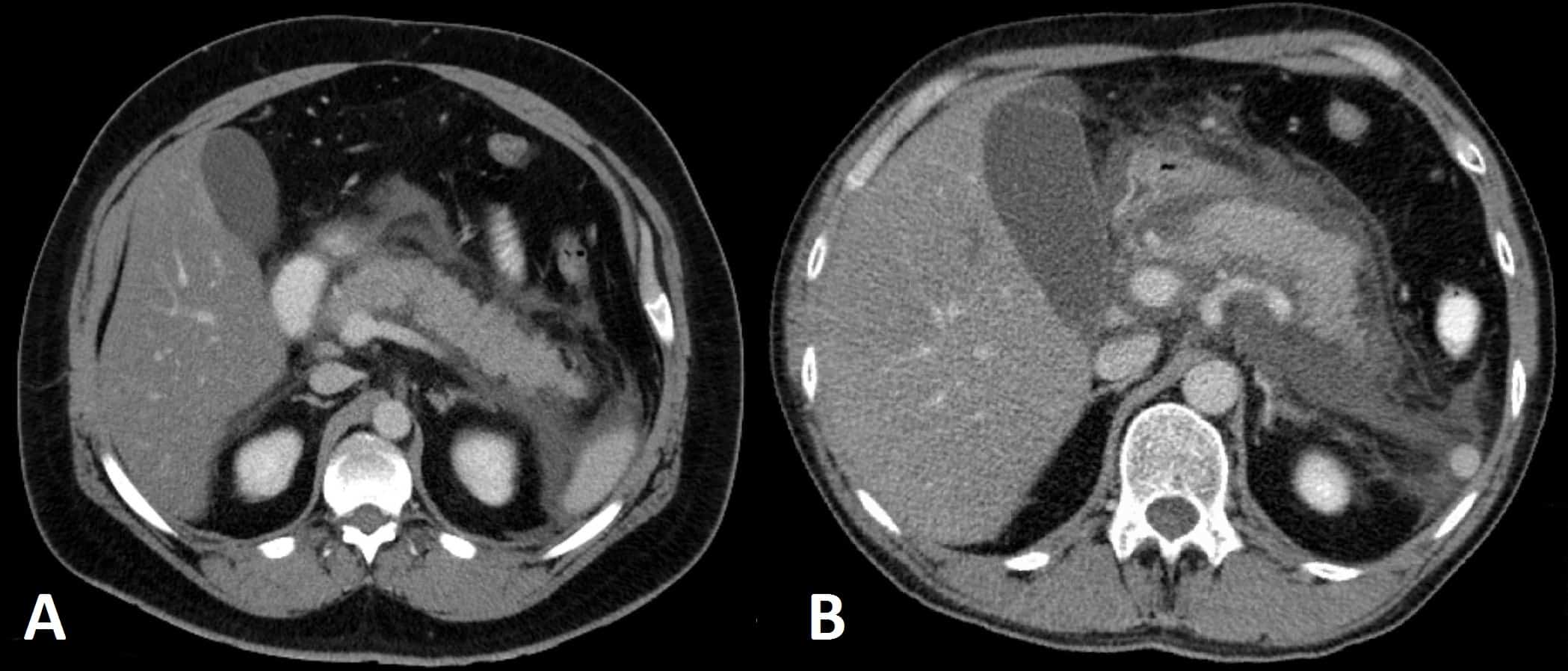
A contrast-enhanced CT scan (Fig. 3) may be required if the initial assessment and investigations prove inconclusive. If performed after 48hrs from initial presentation, it will often show areas of pancreatic oedema and swelling, or any non-enhancing areas suggestive of pancreatic necrosis.
Current UK guidelines state that any CT scan used to assess for severity of disease should only be performed 6-10 days after admission in patients with features of persistent inflammatory response or organ failure*.
*Prior to this time frame, CT-based severity scoring systems have been shown to be equivocal to clinical scoring systems in predicting severity, whilst increasing length of hospital stay with no improvement in clinical outcome
Management
There is no curative management for acute pancreatitis, so supportive measures are the mainstay of treatment. Treat any underlying cause as necessary (e.g. urgent ERCP and sphincterotomy for gallstones) where appropriate.
Supportive treatment includes:
- Intravenous fluid resuscitation, and oxygen therapy as required
- A balanced crystalloid should be used
- Nasogastric tube if the patient is vomiting profusely
- If the patient is able to eat, oral intake can be encouraged as tolerated
- Catheterisation to accurately monitor urine output and start a fluid balance chart (due to the potential for rapid third space losses)
- Aim for a urine output of at least >0.5ml/kg/hr
- Opioid analgesia*
Current UK guidelines state that all patients with severe acute pancreatitis should be managed in a high dependency unit or intensive therapy unit (although this is often impractical).
A broad-spectrum antibiotic, such as imipenem, should be considered for prophylaxis against infection in cases of confirmed pancreatic necrosis.
Treating the underlying cause should be addressed, once the patient has been stabilised. For those caused by gallstones, early laparoscopic cholecystectomy is advised, whilst those secondary to alcohol excess should ensure they have access to the appropriate services made.
*A Cochrane review stated there is no current evidence that suggests opioid analgesia should be avoided due to increased risk of pancreatitis complications or other adverse events when compared to other analgesia
Complications
Systemic Complications
The systemic complications of acute pancreatitis tend to occur within days of the initial onset:
- Disseminated Intravascular Coagulation (DIC)
- Acute Respiratory Distress Syndrome (ARDS)
- Hypocalcaemia
- Fat necrosis from released lipases, results in the release of free fatty acids, which react with serum calcium to form chalky deposits in fatty tissue
- Hyperglycaemia
- Secondary to destruction of islets of Langerhans and subsequent disturbances to insulin metabolism
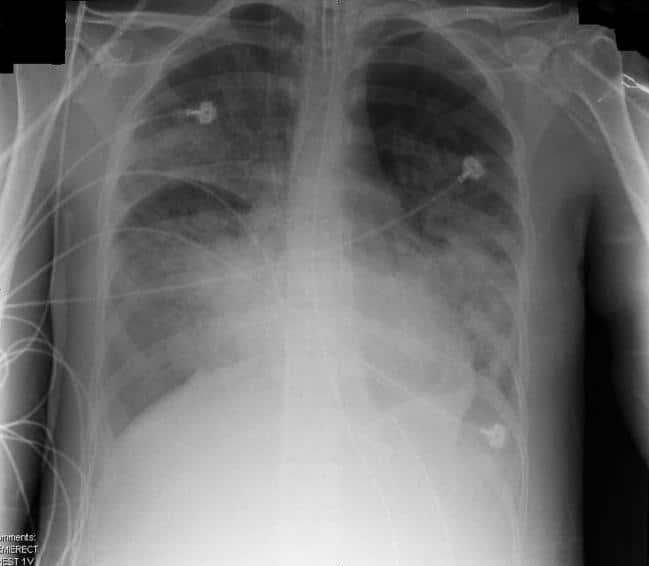
Figure 4 – CXR showing features of acute respiratory distress syndrome, a complication that can occur of acute pancreatitis
Local Complications
Pancreatic Necrosis
Ongoing inflammation eventually leads to ischaemic infarction of the pancreatic tissue, hence such progression should be suspected in patients with evidence of persistent systemic inflammation for more than 7-10 days after the onset of pancreatitis.
Any suspected pancreatic necrosis should be confirmed by CT imaging and treatment will often warrant pancreatic necrosectomy (open or endoscopic)*.
Pancreatic necrosis is prone to infection and should be suspected if there is a clinical deterioration in the patient associated with raised infection markers (or from positive blood culture or changes of low density within the pancreas on CT). Definitive diagnosis of infected pancreatic necrosis can be confirmed by a fine needle aspiration of the necrosis.
*General consensus for intervention in cases of confirmed pancreatic necrosis is to be delayed until walled-off necrosis has developed, typically 3-5 weeks after the onset of symptoms
Pancreatic Pseudocyst
A pancreatic pseudocyst is a collection of fluid containing pancreatic enzymes, blood, and necrotic tissue; they can occur anywhere within or adjacent to the pancreas, however are usually seen in the lesser sac obstructing the gastro-epiploic foramen by inflammatory adhesions..
They are typically formed weeks after the initial acute pancreatitis episode. They lack an epithelial lining, therefore termed pseudocyst, and instead have a vascular and fibrotic wall surrounding the collection.
Pseudocysts may be found incidentally on imaging or can present with symptoms of mass effect, such as biliary obstruction or gastric outlet obstruction. They are prone to haemorrhage or rupture, and can become infected.
About 50% will spontaneously resolve, hence conservative management is usually the initial treatment of choice. Cysts which have been present for longer than 6 weeks are unlikely to resolve spontaneously. Treatment options include surgical debridement or endoscopic drainage (often into the stomach).
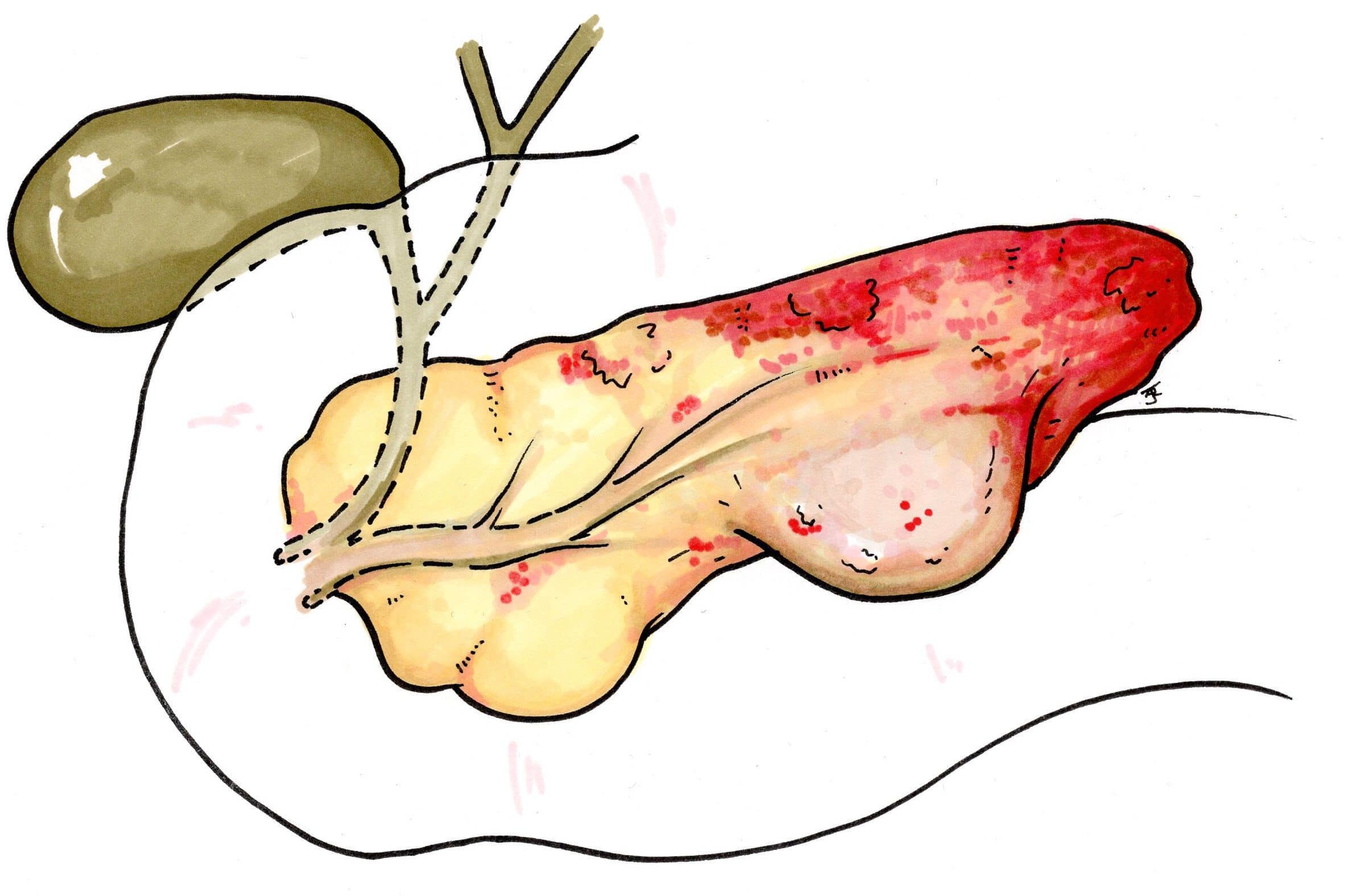
Figure 5 – Schematic demonstrating a pancreatic necrosis
Key Points
- Most cases of acute pancreatitis are due to either gallstones or alcohol
- Serum amylase 3 times the upper limit of normal is diagnostic of acute pancreatitis
- Abdominal US scans are used to investigate the potential underlying causes whilst CT scans are only used if complications are suspected or the diagnosis is not certain
- Treatment is conservative and antibiotics should only be used as prophylaxis in cases of confirmed pancreatic necrosis