Introduction
Valvular disease is common amongst the general population, however fortunately the vast majority is mild and does not need intervention. The prevalence is around 2.5% in the general population, however rises to over 10% in those aged over 75yrs.
The mainstay of diagnosis for valvular disease is with echocardiogram (ECHO), which can not only diagnose the condition but also assess severity and underlying aetiology, and determine the concurrent cardiac function.
Plain film chest radiographs (CXR) and electrocardiograms (ECGs) can be used to look for complications of valvular disease, such as Atrial Fibrillation, and should be performed along with assessment of the coronary system (via either angiography or CT angiograms) prior to any further intervention.
Mitral Regurgitation
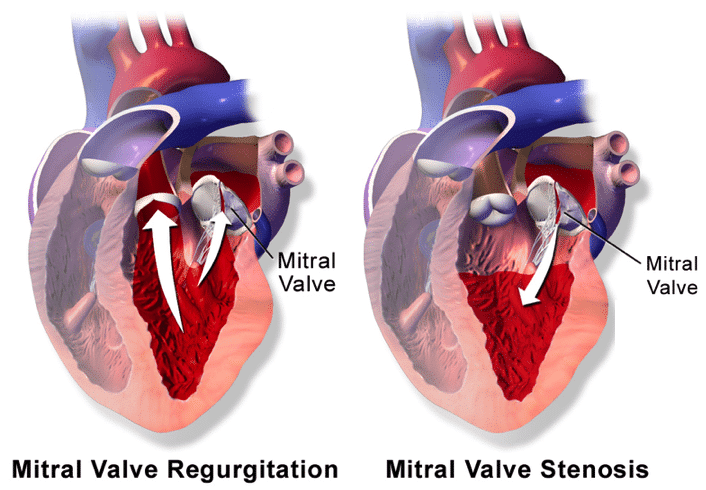
Mitral regurgitation (MR) is characterised by the retrograde flow of blood through the mitral valve from the left ventricle into the left atrium. It is the most common valvular abnormality worldwide.
The causes of MR may be primary, where there is a structural deformity within the valve and its surrounding structures, or secondary (e.g. ischaemic), where there is no structural abnormality of the valve itself. The most common worldwide* cause of MR is mitral valve prolapse secondary to myxomatous degeneration.
*In developing countries, however, rheumatic heart disease remains the most common cause of MR
Classification of Mitral Regurgitation
The Carpentier classification is commonly used to classify the cause of MR according to valve leaflet motion:
- Type 1 – normal leaflet motility, e.g. annular enlargement or perforated leaflet
- Type 2 – increased leaflet motility, e.g. elongated or ruptured chords
- Type 3 – restricted motility, e.g. Rheumatic Heart Disease or ischaemic cardiomyopathy
The retrograde flow of blood across the mitral valve into the left atrium increases the preload of the left ventricle during diastole. The left ventricular stroke volume is thereby increased (as per Starling equation), and in chronic cases this results ventricular dilatation and progressive widening of the annulus of the mitral valve. If left untreated, there is a resultant reduced left ventricle ejection fraction and heart failure.
Patients with MR most often present with features of heart failure (dyspnoea, orthopnoea, paroxysmal nocturnal dyspnoea). On examination, MR patients will have a pansystolic murmur, best heard at the mitral region (cardiac apex) with radiation into the left axilla. Surgical management is reserved for those with symptomatic severe MR.
Mitral Stenosis
Mitral stenosis is caused by the narrowing of the mitral valve orifice, most commonly caused by rheumatic fever, with symptoms typically presenting approximately 10 years after the initial infection.
The narrowing of the mitral valve orifice leads to the formation of a pressure gradient across the valve, increasing atrial pressure and resulting in left atrial dilatation, often also precipitating an atrial fibrillation. The decompensation of the left atrium results in reduced cardiac output and congestive heart failure.
Patients present with features of heart failure, or less commonly from the atrial fibrillation. On examination, a low-pitched, rumbling mid-diastolic murmur is auscultated, loudest in the mitral region and accentuated with the patient in the left lateral decubitus position. The murmur may be accompanied by a loud S1 or an opening snap.
Aortic Stenosis
Aortic stenosis (AS) affects 2-7% of people over 65yrs. It is most commonly caused by age-related calcification, however can be caused by congenital bicuspid valves (in those aged <50yrs) or rheumatic heart disease (especially in developing nations)
Stenosis of the aortic valve results in reduced blood flow from of the left ventricle. Increased resistance results in hypertrophy of the left ventricle (LV), in attempt to maintain the stroke volume. However, eventually the LV will decompensate, leading to heart failure.
In cases of symptomatic aortic stenosis, patients present with exertional dyspnoea, angina*, and syncope (this triad rarely actually occurs together), and can progress to features of heart failure. On examination, an ejection systolic murmur will be present, loudest in the aortic region; patients have a slow-rising pulse with a narrow pulse pressure.
Indications for surgical management are severe symptomatic AS or severe AS with left sided heart failure.
*Angina occurs due to the increasing oxygen demand of the severely hypertrophied left ventricle
Aortic Regurgitation
Aortic regurgitation involves the retrograde flow of blood through the aortic valve from the aorta into left ventricle (Fig. 3). This regurgitation results initially with LV hypertrophy, in order to maintain a stroke volume, however this eventually results in LV dilatation and subsequent heart failure.
The most common reasons for AR are rheumatic fever (most common worldwide), bicuspid valves (most common in developed countries), connective tissue disease, or in acute cases, infective endocarditis, or aortic dissection.
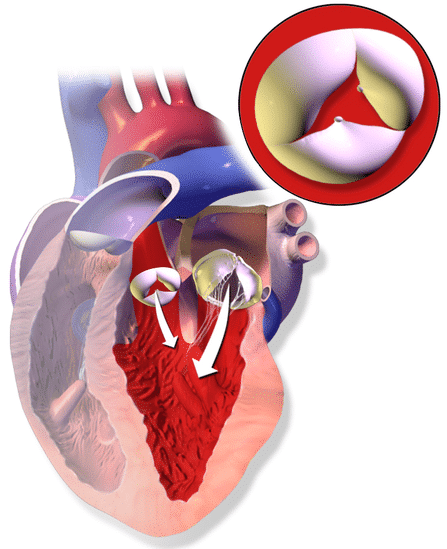
Figure 3 – Illustration demonstrating Aortic Regurgitation
Patients with aortic regurgitation can remain asymptomatic, with the most common late presenting symptom being exertional dyspnoea. On examination, an mid-diastolic murmur can be auscultated (“Austin Flint murmur”) that is loudest in the apex region, a wide pulse pressure is present, and the presence of a collapsing pulse (a “water hammer” pulse)
Eponymous Signs in Aortic Regurgitation
There are multiple eponymous signs that may be identifiable in cases of severe aortic regurgitation:
- Quincke’s sign – visible capillary pulsation of nail bed
- De Musset’s sign – head nodding in time with the heart beat
- Corrigan’s sign – visible arterial pulsation in the neck
- Traube’s sign – “pistolshot” sound upon auscultation of the femoral artery
- Muller’s sign – a pulsatile uvula
Surgical Management
Catheter-Based Interventions
The mainstay of catheter-based interventions is the Transcatheter Aortic Valve implantation (TAVI) procedure. However, newer techniques are being developed for those not deemed fit enough for open surgery and where a TAVI would not be suitable.
For example, for patients with mitral regurgitation a device which clips together the mitral valve leaflets to reduce leakage, called the Mitraclip, has been developed for use in patients who are not deemed fit for conventional open heart surgery.
Transcatheter Aortic Valve implantation
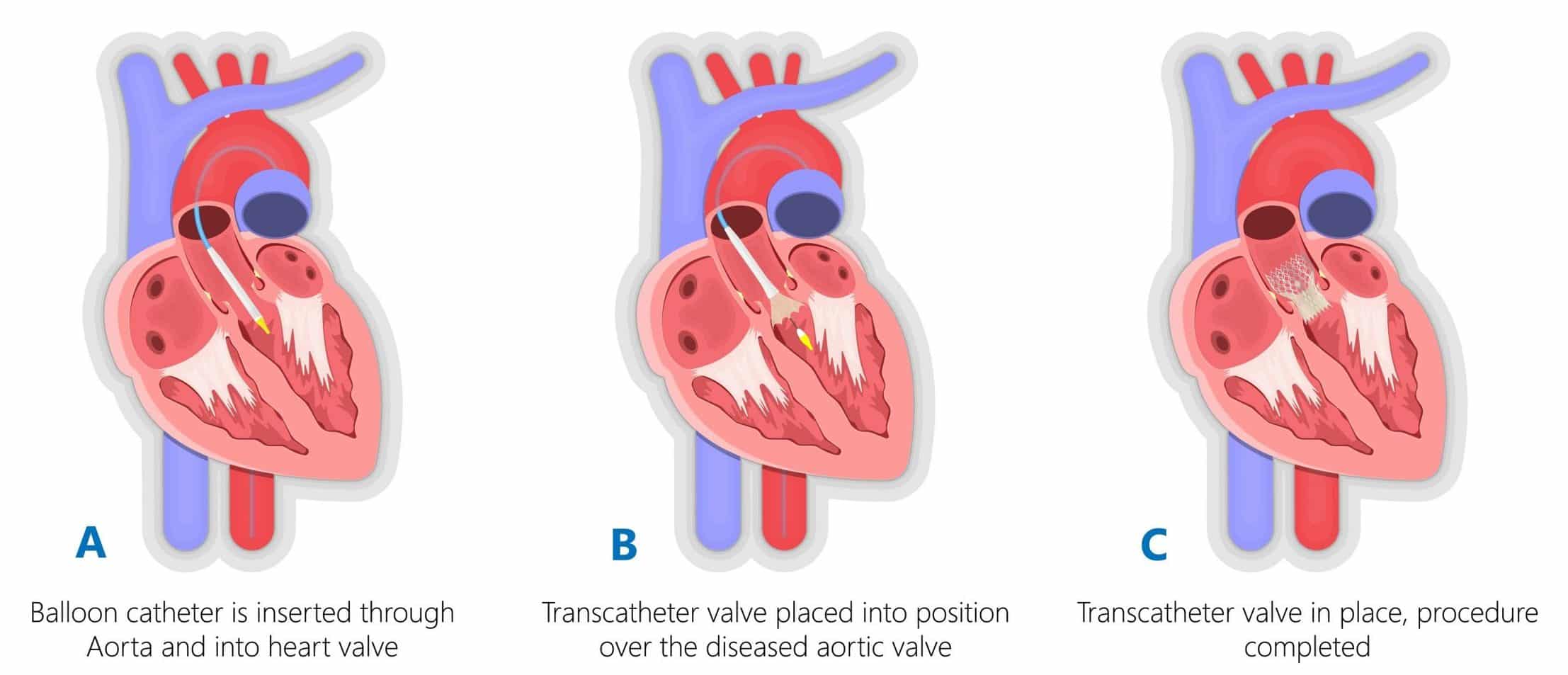
Transcatheter Aortic Valve implantation (TAVI) is a minimally invasive procedure that can be used to treat aortic valvular disease, especially in patients for whom open procedures would be too high risk. The procedure is performed under local anaesthesia, therefore allowing higher risk patients to undergo intervention and leading to (relative to open surgery) shortened hospital stays.
The procedure is performed under X-ray fluoroscopic guidance, with access to the aortic valvular region obtained by inserting a guidewire via the femoral artery. A sheath carrying the collapsed heart valve is then passed over the guidewire into position over the aortic valve (Fig. 4), to then be deployed (either via balloon or a self-expanding mechanism) over the existing valve. Once the valve is completely deployed, an ECHO is performed to assess the new valve’s function and check for perivalvar leakage.
Open Heart Surgery
In open heart surgery, there are a number of different approaches to fixing valvular disease. In valve regurgitation (especially mitral), the ideal operation is valve repair, however replacement can be performed if this is not possible. In valve stenosis, the treatment is typically valve replacement.
Mechanical valves or tissue valves can be used; mechanical valves tend to be lifelong but also require lifelong anticoagulation, whilst tissue valves tend to have a shorter lifespan (degenerating at 10-15 years) but do not require anticoagulation.
The longer-lasting mechanical valves are preferred in younger patients (Table 1), however the patient’s bleeding, lifestyle factors, and patient preference all influence the choice.
|
|
Mechanical |
Tissue |
| Age group | Younger (<60) | Older (>70) |
| Durability | Longer (Lifelong) | Shorter (10-15 years) |
| Anticoagulation | Required (initially heparin, then warfarin lifelong) | Not required |
Table 1 – Factors for Each Valve Type
Open Heart Procedure
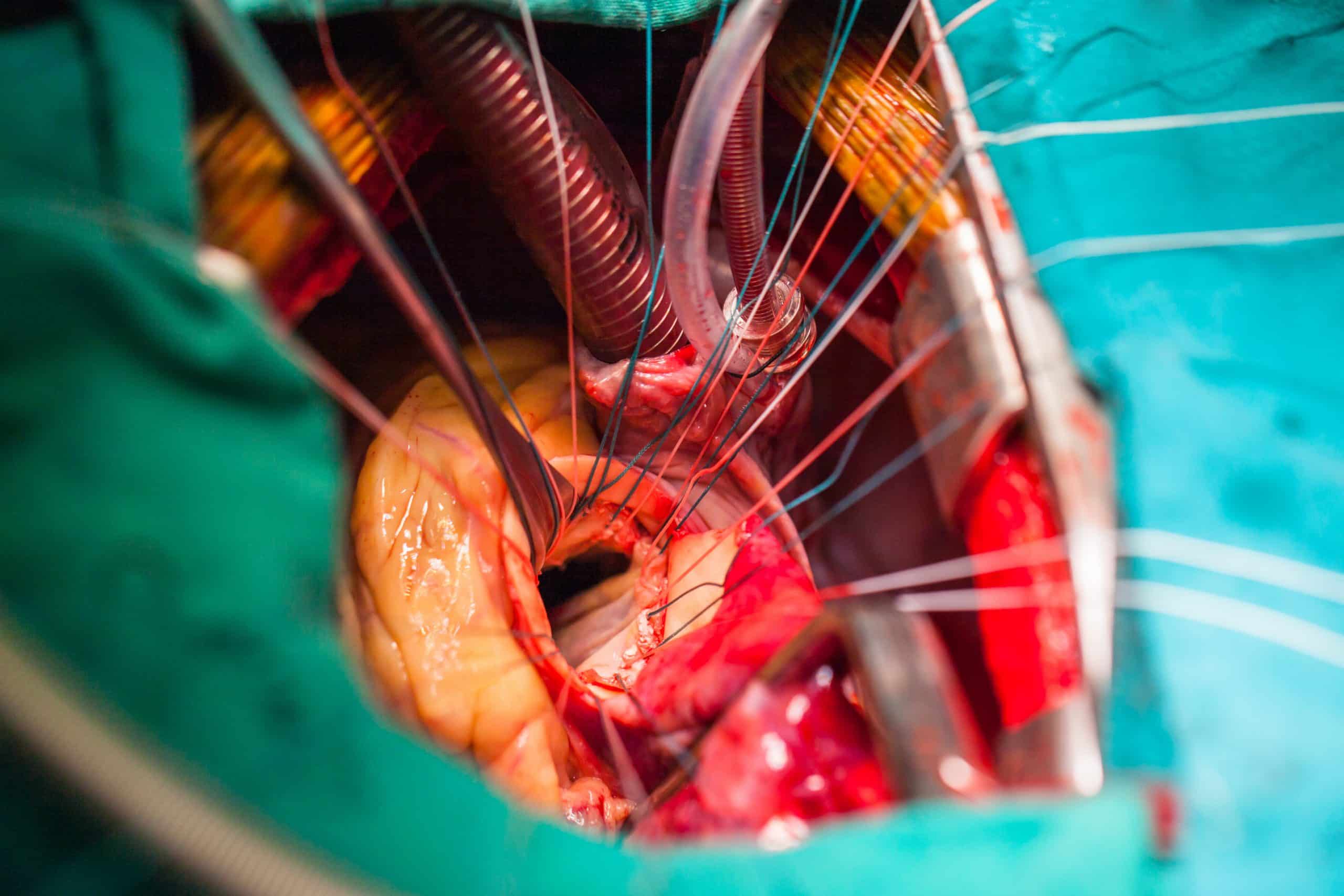
There are many surgical approaches to valve replacement, depending on the location of the valve, extent of disease, anatomical variation, and surgeon’s preference. Traditionally, the heart is accessed via median sternotomy, however less invasive approaches, such as hemi-sternotomies and minithoracotomies are now being increasing used. The patient is then placed on cardiopulmonary bypass.
Access to the valve is obtained, typically via an incision through the ascending aorta in an aortic valve replacement or through the left atrium in mitral valve replacement. Stenotic aortic valves are then dissected away from the annulus and a new valve is then sutured onto the annulus. In mitral regurgitation, the valve is instead ideally repaired, typically with a ring to reduce the size of the annulus and replacement of any ruptured chordae.
The cardiac incision is then closed and transoesophageal ECHO is performed to assess the valve function and peri-valvular leak prior to closure. Transcutaneous pacing wires are inserted to treat any (often transient) heart block which can occur after the procedure, and mediastinal chest drains are inserted to reduce the risk of tamponade. The median sternotomy is then closed using stainless steel wires.
Key Points
- Valvular disease is common amongst the general population, with prevalence increasing with age
- The mainstay of diagnosis for valvular disease is with echocardiogram
- Intervention for valvular disease can be either Catheter-Based Interventions or Open Procedures, depending on a number of patient and surgical factors