One of the biggest barriers in transplantation is the immune response against grafted tissues and thus controlling the immune response is key to successful transplantation.
This article will provide an overview of the key concepts of transplantation immunology and the measures in place in clinical practice to prevent rejection.
Overview of the Immune Response
The immune system is composed of innate and adaptive immune responses.
Innate Immune System
The innate immune system is the fast acting and stereotyped immune response seen in areas where the physical barriers have been breached by microbes.
The innate immunity cells recognise different microbes by the structural groups the pathogen share, known as pathogen-associated molecular patterns (PAMPs), as well as any signals received from stressed or necrotic cells, known as damage-associated molecular patterns (DAMPs). The innate immune cell receptors for these are known as pattern recognition receptors (PRRs), with examples such as the Toll-Like Receptors (TLRs).
Figure 1 – A neutrophil, as seen on a blood film
The innate immune system not only recognises and responds to microbes, but also communicates with the adaptive immune system to respond to different microbes in the most effective manner. The innate immune system is formed of both cellular and humoral components:
- Cellular = neutrophils, natural killer (NK) cells
- Humoral = complement system
The cells of the innate immune system kill target microbes via phagocytosis. Once internalised, the microbe can be destroyed using oxygen-dependent (i.e. reactive oxygen species) or oxygen-independent (e.g. lysosomal enzymes) mechanisms.
Adaptive Immune System
The adaptive immune response is a more specific form of immunity. It can distinguish self from non-self and is has the ability in memory for previously encountered infections.
Cellular Response
The principal cells of adaptive immunity are lymphocytes. T lymphocytes (T cells) come in two main subtypes, either as CD4 T cells (or T helper cells) which stimulate other immune cells by direct interaction or release of cytokines, or CD8 T cells (or cytotoxic T cells) which directly kill infected cells.
All T cells are able to recognise specific peptides derived from foreign proteins when presented via antigen-presenting cells (APCs) using the major histocompatibility complex (MHC) molecules*.
For CD8 cells, once these antigens are displayed via the APCs, they will respond by proliferating and differentiating into effector cells, whose function is to eliminate the antigen, and into memory cells, which show enhanced responses on subsequent encounters with the antigen. CD8 cells induce cell death of infected cells by stimulating apoptosis.
*Human MHC molecules are called human leukocyte antigens (HLA), and in the context of human transplantation, the terms MHC and HLA are used interchangeably.
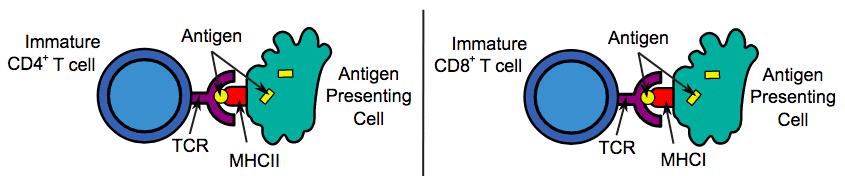
Figure 2 – The antigen presenting cells acting to stimulate and activate CD4+ and CD8+ T cells
Humoral Response
The humoral response of the adaptive immune system is mediated by antibodies, produced by cells called B lymphocytes (often termed B cells). Humoral immune responses are initiated when antigen-specific B lymphocytes in the spleen, lymph nodes, or mucosal lymphoid tissue recognise the antigen (either free in the blood, free in the lymph, or presented directly by a macrophage).
The B cells then proliferative and are activated by CD4 T cells to release specific antibodies*. Antibodies can act to:
– Stimulates complement activation (via the classical pathway)
– Opsonisation for microbes, to allow for improved pathogen phagocytosis
– Neutralisation of certain microbes and toxins
– Allowing for antibody-dependant cellular cytotoxicity (ADCC) of NK cells
*B cells can form memory cells that can rapidly respond to re-exposure to the antigen by proliferating and rapidly secreting high-affinity antibodies.
Immune Response to Allografts
In the field of transplantation, the antigens that stimulate adaptive immune responses against allografts are histocompatibility proteins; the MHC molecules are the most important, responsible for the strong and rapid rejection reactions.
When lymphocytes recognise alloantigens (non-self antigens), they proliferate, differentiate, and damage grafts through cytokine-mediated inflammation (via CD4 T cells) or cell lysis (via CD8 T cells). B cells are also recruited via CD4 T cell stimulation, therefore antibodies against graft antigens can also contribute to rejection.
Although MHC molecules are the strongest barrier to transplantation, there are many other histocompatibility molecules present. The presence of a number of minor histocompatibility molecules explains why certain grafts fail even though they have a perfect MHC match.
Allograft Rejection
There are three types of graft rejection that can occur, defined as hyperacute, acute, and chronic rejection.
Hyperacute Rejection
Hyperacute rejection occurs within minutes to hours. It is observed typically with xenogeneic grafts (those between different species) or, in rare instances, following ABO incompatibility in allogeneic grafts. Hyperacute rejection leads to graft destruction within 24 hours.
It occurs due to pre-existing antibodies directed at components of the graft, usually the endothelial cells, leading to rapid activation of the innate and adaptive immune system. The ensuing endothelial injury leads to platelet adhesion and thrombosis, thus any graft encountering hyperacute rejections never becomes vascularised.
Acute Rejection
Acute rejection occurs over a period a few days following transplantation, either cell‑mediated (more common) or antibody‑mediated immunity.
Cell-mediated acute rejection typically results in a necrosis of parenchymal cells in the graft and is associated with lymphocyte and macrophage infiltrates (Fig. 3). Antibody‑mediated acute rejection leads to necrosis of individual endothelial cells and to a vasculitis-type response.
Treatment usually initially involves high-dose intravenous methylprednisolone, followed by plasma exchange or intravenous immunoglobulin in non-responding cases.

Figure 3 – Histology of a kidney graft biopsy with acute cellular rejection
Chronic Rejection
Chronic rejection occurs after weeks or months post-transplantation and is characterised by fibrosis and arteriosclerosis, especially common in kidney and heart transplants.
The pathophysiology for chronic rejection is more variable yet is often due to extensive intimal proliferation of smooth muscle cells. Currently there are no effective therapeutic strategies for chronic rejection and, as a result, patients may require re-transplantation.
Management of Allograft Rejection
The strategies used in clinical practice to avoid or to delay rejection are optimising immunosuppression (see article on Modern Immunosuppression) and reducing the immunogenicity of allografts.
Reducing graft immunogenicity involves minimising the alloantigenic differences between the donor and recipient, which can be done through a variety of ways:
- ABO blood typing – Ensuing ABO matching is particularly important in renal and cardiac transplantation
- Tissue typing – The determination of HLA alleles expressed on donor and recipient cells
- Often matching HLA-A, HLA-B, and HLA-DR are the most important alleles to consider and optimise; zero-antigen mismatches predict the best survival of donor grafts
- Donor specific antibodies (DSA) – Detection of preformed antibodies in the recipient that could recognise donor HLA molecules
- This is usually only performed for renal transplantation
Key Points
- The immune system is divided into innate and adaptive immune systems, both comprising of cellular and humoral responses
- Three types of graft rejection can occur, hyperacute, acute, or chronic rejection
- Both optimising immunosuppression and reducing the immunogenicity of allografts can help to reduce the risk of graft rejection
