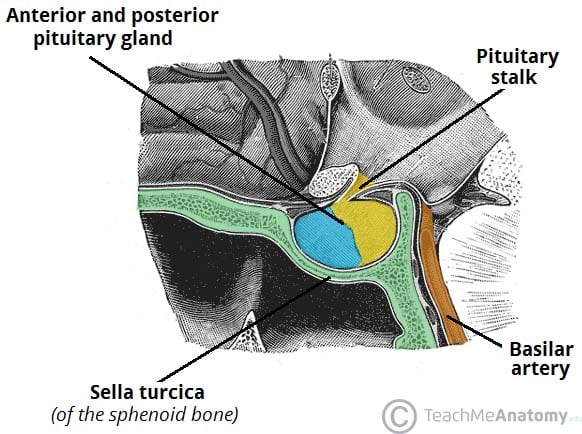
Introduction
Pituitary tumours account for about 8-10% of intracranial tumours*. They typically arise from the anterior lobe of pituitary gland and are predominantly benign adenomas (around 65%).
There are several associated conditions with the development of pituitary tumours, most notably as part of multiple endocrine neoplasia (MEN) syndrome type 1, Carney Complex, and McCune-Albright Syndrome
*Non-secretary microadenomas found incidentally are common; around 10-15% of post-mortem subjects have been shown to have an incidentaloma (a non-secreting microadenoma)
Classification
Pituitary tumours can be classified both by their size and by any hormone produced; histological classification is rarely used in current practice.
When categorised by size:
- Microadenoma = <1cm diameter
- Macroadenoma = >1cm diameter
- Giant adenomas = prolactinoma with >4cm diameter
Any hormone produced by the adenoma is also used to further classify, and can include (from most to least common) prolactin, growth hormone, no hormone secreted, ACTH, gonadotropin (rare), and TSH (rare).
Clinical Features
Patients will present with pituitary adenomas either through features of mass effect and / or from the hormonal effects (hyper- or hypo-secretion) of the tumour.
The growth of the pituitary adenoma can cause a mass effect. Patients can present with non-specific headaches, visual field defects (classically a bitemporal hemianopia, as growth superiorly leads to compression of the optic chiasm), or cranial nerve palsies (through compression on the cavernous sinus (affecting cranial nerves V1, V2, III, IV, and VI).
Rarely, cases may even present with CSF rhinorrhoea (as the tumour erodes through the floor of the pituitary fossa) or pituitary apoplexy.
Pituitary Apoplexy
Pituitary apoplexy is the spontaneous haemorrhage into a pituitary tumour and is a neurosurgical emergency.
Patients present with a sudden and severe headache, loss of consciousness, neck stiffness, vomiting, and / or photophobia.
In suspected cases, IV hydrocortisone cover should be given and consideration for emergency resection of the lesion, to prevent any further visual deterioration and vision loss.
Pituitary tumours can also present from the effects of over or under secretion of the pituitary hormones (Table 1)
| Hormone | Clinical Features | |
|---|---|---|
|
Hypersecretion |
Hyposecretion |
|
| Prolactin | Infertility, galactorrhoea, amenorrhoea (women) / impotence (men) | Failure of lactation (rare) |
| Adrenocorticotropin (ACTH) | Weight gain, fatigue, purple striae, osteoporosis, hypertension* | Fatigue, skin pigmentation, vomiting, weight loss; features of an Addisonian crisis (rare) |
| Growth hormone (GH) | Acromegaly in adult and gigantism in children | Fatigue, reduced strength, weight gain. Dwarfism in children. |
| Gonadotropins (FSH and LH) | Usually asymptomatic | Amenorrhoea, infertility, loss of libido |
| Thyroid-stimulating hormone (TSH) | Palpitations, anxiety, weight loss, heat intolerance, shortness of breathØ | Fatigue, constipation, weight gain, hair loss, swelling of extremitiesØ |
Table 1 – Clinical Features of Hormone Hyper- or Hypo-secretion from Pituitary Adenomas; *often termed Cushing’s disease; ØSecondary hyper- / hypo-thyroidism
Differential Diagnosis
The main differential diagnosis to consider is a craniopharyngioma (derived from pituitary gland embryonic tissue), which can be distinguished by appearances on imaging (typically have a cystic appearance with a supracellar origin).
Other differentials include meningioma, cerebral metastasis, or arachnoid cysts (located around the optic nerve or hypothalamus, rare)
Investigations
Initial diagnosis is made radiologically, through either a high-resolution CT scan or a MRI scan; MRI imaging is deemed more sensitive than CT in identifying microadenoma and is gold-standard investigation.
All hormones potentially affected by a suspected pituitary tumour should be tested for:
- Prolactin – Serum prolactin >2000 mU/l is indicative of a prolactinoma (however other causes for hyperprolactinaemia must be excluded)*
- Growth Hormone – Insulin-like growth factor 1 (IGF-1) as the screening test, then confirmed by failure to suppress growth hormone levels in a glucose tolerance test is diagnostic
- Adrenocorticotropin Hormone – confirm raised cortisol levels (via 24-hour urine cortisol), then check plasma ACTH levels, then confirm pituitary source via short Synacthen test
- Gonadotrophins – Check LH and FSH levels
- Thyroid Stimulating Hormone – check the thyroid function tests
*Causes of a falsely raised prolactin level include stress, pregnancy, breast, certain antipsychotics and SSRIs, hypothyroidism, CKD, and status epilepticus
Management
Treatment planning is based on the effects of the tumour (symptomatic or asymptomatic), patient co-morbidities, and the patient preference.
Conservative Management
Hormone replacement needs to be started as soon as possible* for cases of pituitary adenoma with hyposecretion.
Some pituitary tumours may be sensitive to drug therapy, especially useful for those not suitable for surgery. Prolactinoma can be managed by via dopamine agonists (e.g. cabergoline), reducing both prolactin levels and the size of the tumour. Somatostatin analogues (e.g. ocreotide) and GH receptor antagonists can also be used to inhibit GH seceretion.
*Corticosteroids should be started before thyroxine (if both required), as thyroxine can precipitate an adrenal crisis
Surgical Management
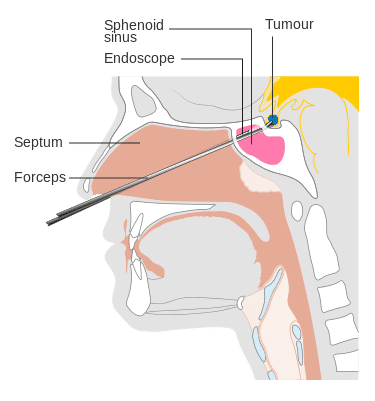
Figure 3 – Diagram demonstrating the access required for trans-spenoidal surgery
Surgery is the mainstay of treatment for pituitary adenomas. The most common approach is trans-sphenoidal (sub-frontal, trans-cavernous, or trans-callosal approachs can be used), whereby the sella turcica is removed and a formal durotomy is performed, to allow the removal of the sellar mass.
Post-operative complications include CSF leak and meningitis, bleeding, and endocrine disturbances; diabetes insipidus is an important complication to be aware of due to disruption of the hypothalamic axis with the posterior pituitary. Pituitary function needs to be re-evaluated post-operatively to identify any needs for hormone replacement.
Highly focal radiotherapy, known as stereotactic radiosurgery (cyberknife/ gammaknife), can be used to control residual tumour or recurrences.
Key Points
- Pituitary tumours are typically benign adenomas that arise from the anterior lobe of pituitary gland
- They can be classified based on both size and hormone secretion status
- Patients present with either features of mass effect and / or hormone imbalance
- Diagnosis is through radiological assessment and hormone profile
- Definitive management is surgical, however conservative management options are available