Introduction
Subarachnoid haemorrhage (SAH) is bleeding into the subarachnoid space, the anatomical space between the arachnoid mater and pia mater (Fig. 1).
SAH can be classified into either aneurysmal or non-aneurysmal causes:
- Aneurysmal disease (85%) is associated with ADPKD, fibromuscular dysplasia, connective tissue disorders, atherosclerosis, and hypertension
- Non-aneurysmal causes for SAH include trauma, arteriovenous malformations, coagulopathies, or tumour-related
They typically occur in patients around 60yrs old and account for 3% of all strokes.
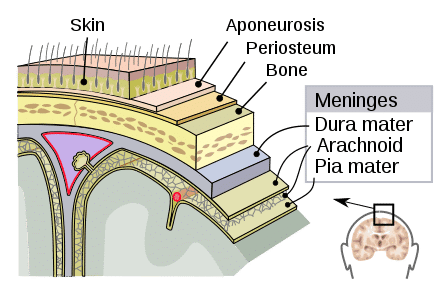
Figure 1 – The layers of the meninges
Berry Aneurysms
Berry aneurysms are ‘berry-shaped’ true aneurysms that occur at the bifurcation of arteries.
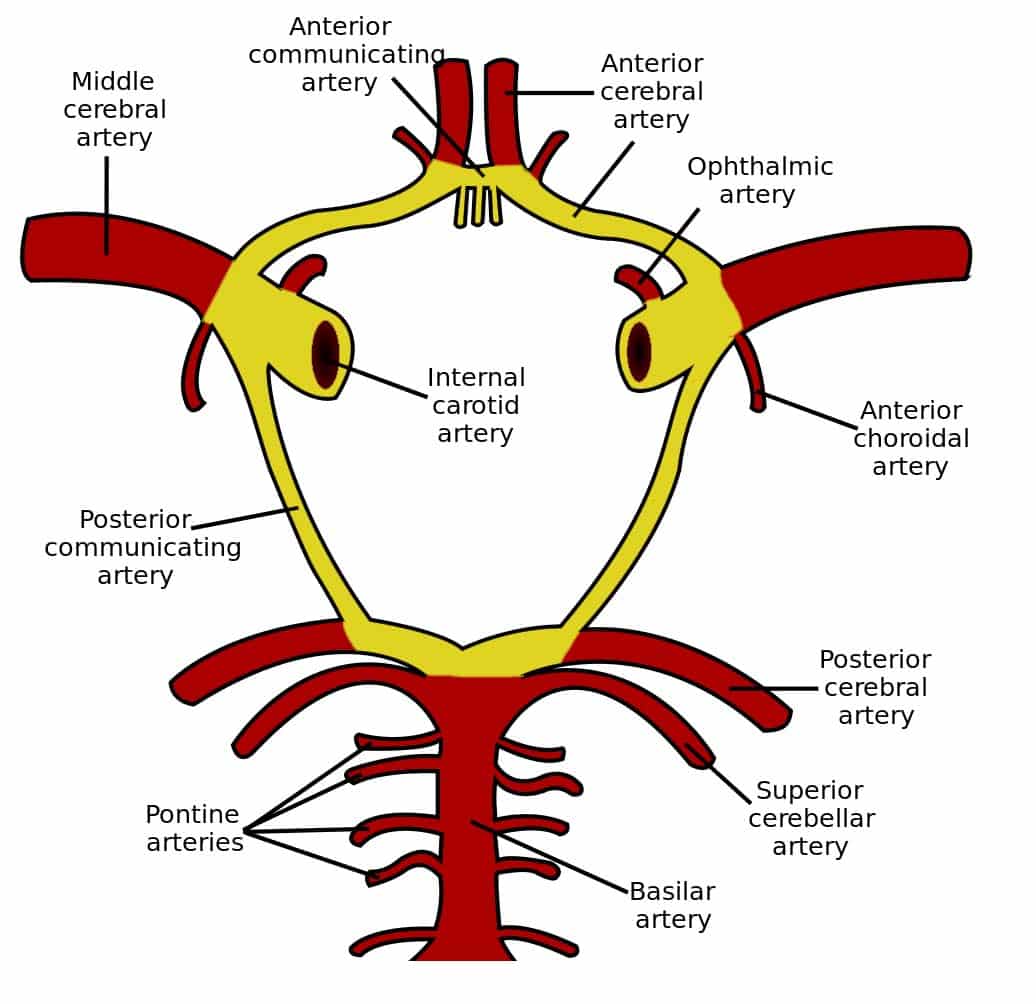
They are typically saccular aneurysms that occur mostly at either the circle of Willis and the bifurcation of the middle cerebral artery (Fig. 2).
Unruptured aneurysms occur in around 3% of the adult population; those that present ruptured will be at the age 40-60yrs and can cause either a subarachnoid haemorrhage, cerebral haematoma, or intraventricular haemorrhage.
Clinical Features
SAH will classically presents with a severe headache, sudden onset (within seconds to minutes), typically in the occipital region (often termed “thunderclap” headaches).
Other features include nausea and vomiting, reduced consciousness, collapse, or seizures, or evidence of meningism (including photophobia, stiff neck, pain on neck flexion, or positive Kernig’s sign).
Examination may reveal focal neurology or evidence of meningism, however may be otherwise unremarkable.
Investigations
Investigations for a suspected SAH should aim to initially confirm the presence of subarachnoid haemorrhage and then to localise the source of any potential bleeding to decide on a management plan
Initial Investigations
An initial urgent non-contrast CT head scan is typically warranted for assessment for SAH (Fig. 4), with a sensitivity of 98% within 12 hours (however small SAH may not be picked up). Hyperattenuating material is seen within the subarachnoid space, most apparent typically around the circle of Willis* or in the Sylvian fissure.
In cases where the initial CT is negative however clinical history is suggestive of SAH, then a lumbar puncture (LP) may be warranted. CSF spectrophotometric analysis following LP will reveal elevated levels of oxyhaemoglobin and bilirubin, in cases of SAH.
*This occurs due to the majority of berry aneurysms occurring in this region

Figure 4 – A non-contrast CT showing a hyperintense area in the subarachnoid basal cisterns, in keeping with subarachnoid haemaorrhage, with widening of the temporal horns of the lateral ventricles suggestive of an associated hydrocephalus
Definitive Imaging
Once the SAH has been confirmed, further imaging is warranted to localise the source of bleed.
Several potential modalities are available, including CT angiography or digital subtraction angiography (which allows for better visualisation of the smaller vessels and the feeding vessels), depending on the treating centre.
Management
The mainstay of treatment is to definitively treat the bleeding region and reduce potential complications.
All acutely unwell patients should be stabilised using an A to E approach, with fluid resuscitation and analgesia with anti-emetics as necessary. Once the condition is identified, early neurosurgical involvement is essential and transfer to a high-dependency care centre.
Patients can be started on nimodipine, a calcium channel blocker that aims to reduce vasospasm and reduce the risk of delayed cerebral ischaemia
Surgical Management
Ruptured aneurysmal SAH will commonly require neurosurgical intervention, either coiling or clipping of the aneurysm, depending on the location, size, age, and comorbidities.
Nearly 80% of aneurysms are coiled (Fig. 5), however this requires a suitable aneurysm neck to dome ratio. Coiling is more suitable in the presence of multiple co-morbidities, older patient age, presence of vasospasm, or aneurysms present in deep areas or along the basilar artery.
Clipping is now currently only performed in a minority of aneurysms, mainly when aneurysm morphology is not suitable for coiling (e.g. wide necked aneurysms), there is a presence of a clot, or a previously coiled aneurysm has ruptured.
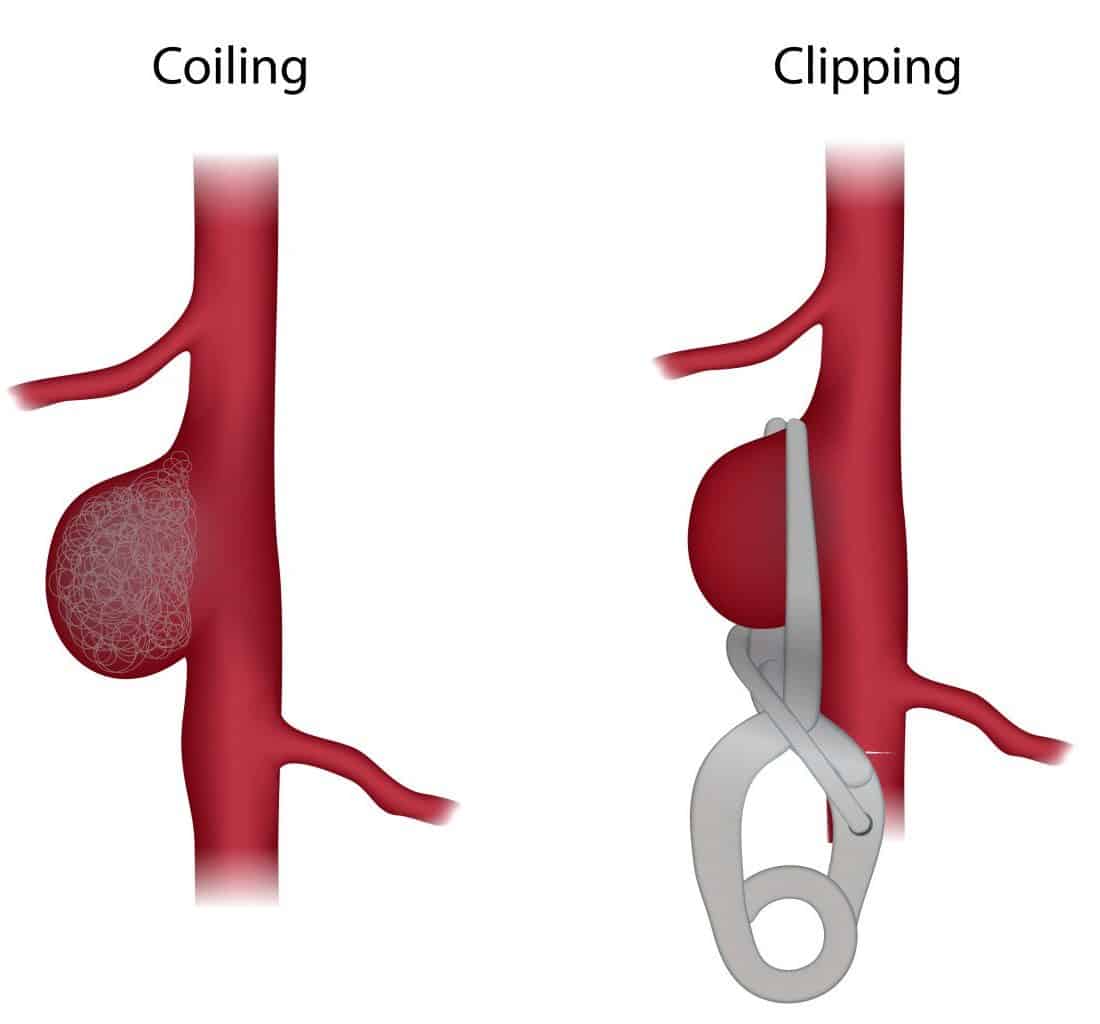
Figure 5 – Illustration demonstrating aneurysm coiling vs clipping
Ongoing Monitoring
Patients need close monitoring for potential complications, including rebleeding, hydrocephalus, vasospasm, and electrolyte disturbances (particularly hyponatremia).
Patients may warrant ICP monitoring. Prophylactic levetiracetam may be started to reduce seizure risk.
Key Points
- Subarachnoid haemorrhage is bleeding into the subarachnoid space, most commonly caused by ruptured Berry aneurysms
- Patients will typically present with severe sudden-onset occipital headache, with evidence of meningism and reduced consciousness
- Patients should have a non-contrast CT head scan initially to assess for potential subarachnoid haemorrhage
- Ruptured aneurysms suitable for intervention will usually undergo coling