Introduction
Neuroendocrine cells are any cells that receives input from neurotransmitters to release hormones into the bloodstream, allowing for an integration between the nervous and endocrine systems.
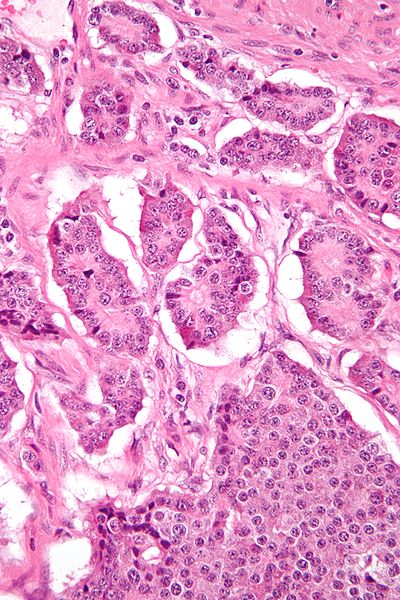
Gastroenteropancreatic neuroendocrine tumours (GEP-NETs) refers to neuroendrocrine tumours originating from neuroendocrine cells in the gastrointestinal tract or the pancreas*, all of which have malignant potential; pancreatic neuroendocrine tumours are discussed further here
The incidence of GEP-NETs is between 2-3 per 100 000 persons per year, with slightly higher rates in women, and rates have been increasing in recent years. The majority of GEP-NETs are located in the small intestine, the remainder either in the rectum or the stomach.
*Neoplasia arising from neuroendocrine cells have historically been called carcinoid tumours when occurring in the gastrointestinal tract, however they can also occur in the pancreas and lung
Risk Factors
NETs may occur as part of complex familial endocrine cancer syndromes such as MEN1, MEN2, neurofibromatosis type 1 (NF1), Von Hippel Lindau (VHL), and Carney complex, however the majority of NETs occur are sporadic. Other risk factors include female gender and family history of NETs.
Clinical Features
GEP-NETs may be classified into non-functioning tumours (the majority), which have no hormone-related clinical features, or functioning tumours, which cause symptoms due to peptide and hormone release.
All GEP-NETs can present with non-specific symptoms, such as vague abdominal pain, nausea and vomiting, and abdominal distension. In less common cases, they may present with features of bowel obstruction (or appendicitis in appendiceal GEP-NETs), requiring urgent intervention
Patients may have concurrent features suggestive of an underlying inherited disorder, such as MEN-1 or VHL
Functioning NETs
The small proportion of functional GEP-NETs can present with additional features from peptide hormone release. Whilst pancreatic NETs can produce a range of hormones, around 20% of well-differentiation small bowel NETs can present with carcinoid syndrome.
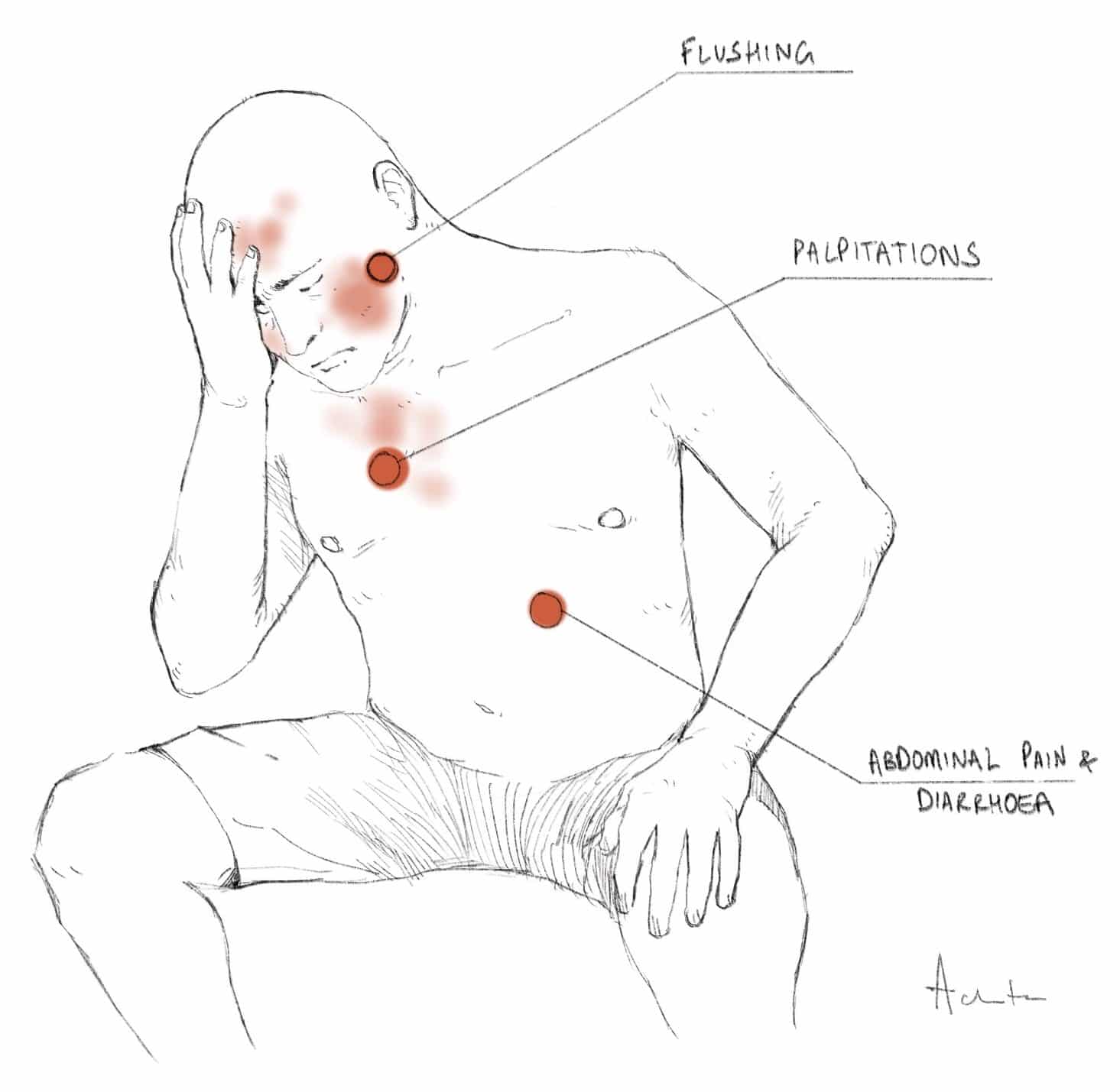
Carcinoid Syndrome
Carcinoid syndrome occurs in advanced carcinoid tumours whereby the metastasised cells begin to oversecrete bioactive mediators, such as serotonin, prostaglandins, and gastrin, into the circulation. The secreted substances are usually metabolized and inactivated by the liver, hence carcinoid syndrome usually occurs in patients with liver metastases, whereby first pass metabolism is bypassed. Patients present with symptoms of flushing, palpitations, intermittent abdominal pain, diarrhoea, and palpitations.
Investigations
Diagnosis of GEP-NETs is based upon clinical features, biochemistry, and specialised radiological imaging
Laboratory Tests
Chromogranin A (CgA) should be tested for all patients, as it is the only general marker for NETs that is found in high concentrations regardless of whether the tumour is functional or non-functional. In cases where CgA is normal, Chromogranin B (CgB) can also be tested.
Pancreatic polypeptide (PP) can be checked in cases where CgA and CgB levels are normal; PP is secreted in high concentrations from a significant proportion of GEP-NETs (50–80% of pancreatic NETs and >30% of all gut NETs).
Some centres will also check urinary 5-hydroxyindoleacetic acid (5-HIAA) levels, a breakdown product of serotonin, however issues can occur with its collection (a 24-hour urine sample) and drug interference, so it is not always routinely tested.
For pancreatic NETs, further specific markers are also checked (see here). Genetic testing should be considered if the clinical history is suggestive.
Imaging
CT imaging is the most widely used initial imaging investigation* for patients with a suspected GI NET involving the small bowel, however its sensitivity is limited, unless lymphadenopathy or liver metastasis are present.
However, CT enterography or enteroclysis imaging combines the cross-sectional display of solid organs with the luminal and mural display of the small bowel, therefore is more sensitive than routine CT imaging in demonstrating an primary GI NET.
Endoscopy remains the investigation of choice for suspected gastric, duodenal, and colorectal NETs, and EUS for pancreatic NETs where possible. Where feasible, biopsy samples should be taken for gold-standard pre-operative diagnosis.
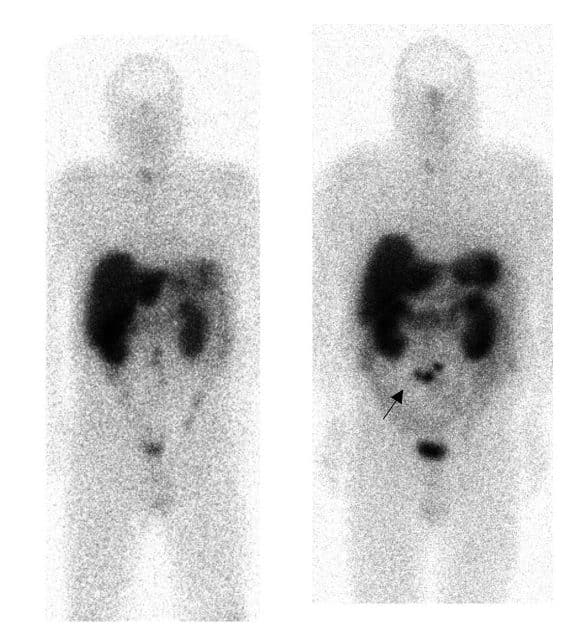
For patients who present with metastatic disease without a known primary (and to establish the extent of disease in those with a known primary), whole body somatostatin receptor scintigraphy (SSRS) can be used (Fig. 3); these use a radioactive tracer that binds to somatostatin receptors, often expressed on NET cells.
Management
All patients should be discussed at a multi-disciplinary team meeting at a tertiary hospital where management can be guided. Surgery is the only curative treatment for GEP-NETs*, however nodal or liver metastases are found at presentation in 40–70% of patients.
Well-differentiated GEP-NETs are managed according to site, staging, and functionality. Localised disease should be resected; any liver metastases present should also be resected where feasible, along with the primary tumour, as this has been shown to improve survival rates.
Poorly-differentiated GEP-NETs have a poor prognosis; if the disease is localised, then treatment is often surgical resection followed by chemotherapy, whilst in metastatic disease, palliative chemotherapy alone is typically advised.
In the presence of liver metastases, curative liver resection is possible in about 10% of cases, if the lesion is confined to one lobe. Another option is radiofrequency ablation of liver metastases.
Newer treatment options include peptide receptor radionuclide therapy and targeted immunotherapies, such as evertolimus or sunitinib (these options need specialised oncology input and would depend on the biology of the NET).
*Symptomatic control for those with carcinoid syndrome can be achieved with use of somatostatin analogues, mainly octreotide or lanreotide
Carcinoid Crisis
When major surgery or embolisation is planned for patients with carcinoid syndrome, prophylactic administration of somatostatin analogues should be considered to prevent a potential carcinoid crisis, both intra-operatively or post-operatively. Carcinoid crisis is caused by an overwhelming release of hormones from the NET, resulting in a resistant severe hypotension.
Somatostatin analogues (such as ocreotide) may be used for the prophylaxis of carcinoid crisis, depending on the presence of carcinoid syndrome, how well it is controlled, and the type and degree of surgery planned. In the highest risk patients, octreotide can be started 24 hours prior to the operation and continued for 48 hours post-operatively.
Surgical Management
Gastric NETs management depend on grade, as the lower subtypes often have a very low metastatic potential, therefore can usually be treated with endoscopic resection and annual surveillance. However, the more aggressive lesions should be treated more aggressively, requiring gastrectomy with regional lymph node clearance.
Small intestinal NETs are almost always malignant. Resection of the tumour with mesenteric lymph node clearance is nearly always performed, regardless of the presence of liver metastases.
Appendiceal NETs are often identified incidentally following appendicectomy. A completion right hemicolectomy will be required for tumours >2cm in size, with a serosal breach, cellular atypia, invasion of mesoappendix (by more than 3 mm), or involvement of the base of the appendix
Colonic NETs have the worst prognosis of any gastrointestinal NET, with their treatment often requiring a segmental colectomy with regional lymph node clearance. Rectal NETs have a more benign character, with smaller tumours treated with endoscopic resection and larger tumours requiring either AP resection or low anterior resection.
Prognosis
NETs are slow growing tumours and prognosis varies on location, with the highest 5-year survival rates in those with rectal NETs (74–88%) and the lowest in those with pancreatic NETs (27–43%). Prognosis also depends on extent of the disease at the time of diagnosis, the degree of differentiation of the tumour, histopathological subtype, and patient age.
The likelihood of nodal involvement at the time of diagnosis in a patient with a small bowel NET is about 60%, and slightly lower for colonic or appendiceal NETs. The chance of liver metastases being present at the time of diagnosis is about half the risk of nodal spread having occurred.
Key Points
- Gastroenteropancreatic neuroendocrine tumours (GEP-NETs) originate from neuroendocrine cells in the gastrointestinal tract and the pancreas
- Any suspected case should be investigated initially via Chromogranin A levels and subsequent endoscopic and CT imaging
- Surgery is the only curative treatment, however many tumours will already have metastasised at presentation