Introduction
Post-operative pain management is a key responsibility of the junior doctor and is one of the most common “bleeps” to receive. Indeed with the careful applications of a few core principles, it can often prove a simple and rewarding management.
Pain can be divided into acute or chronic types. This article will predominantly focus on the assessment and management of acute pain.
Clinical Assessment of Pain
Post-operative pain can be assessed subjectively and objectively:
- Subjective– Ask the patient to grade their pain on a scale of mild, moderate, or severe; this can be assessed regularly as part of the nursing observations.
- Historically, pain has been graded on a severity from 1 to 10*, however this method is often problematic as it requires the patient to quantify their pain into a number and the doctor to then interpret that into an appropriate analgesia regime
- Objective– Clinical features of pain include tachycardia, tachypnoea, hypertension, sweating, or flushing
- An unwillingness to mobilise or agitation may be present in those that are less able to communicate their pain
Each patient should be assessed when mobile, when taking a deep breath, and when in bed (a pain-free patient in bed may well be in severe pain when they walk to the toilet).
Remember that pain itself may be a marker of underlying pathology (not always just “routine post-operative pain”), therefore it is important to assess for any underlying (and potentially treatable) causes for the pain.
*Children may not be able to use this scale and there are pictorial representations of pain to help guide their pain levels
Consequences of Poor Pain Control
Inadequate control of post-operative pain results in a slower recovery. Patients with poorly controlled pain are often reluctant to mobilise, in turn resulting in a slower restoration of function and rehabilitation capacity
As an example, patients in pain following abdominal surgery will not breathe as deeply as they normally would and may not be able to cough to clear secretions resulting in inadequate ventilation and subsequent atelectasis and hospital-acquired pneumonia
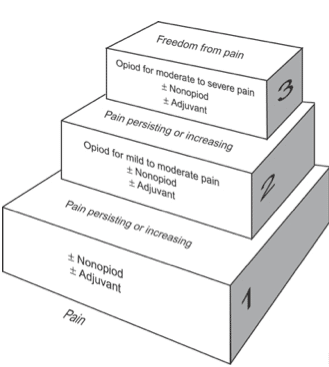
WHO Analgesic Ladder
The World Health Organisation Analgesic Ladder (Fig. 1) is the best-known method for approaching pain relief. It provides a strategy for titrating analgesia, starting with simple analgesics and working upwards to strong opioids. After implementing a regime, the patient should always be reviewed shortly after to assess adequacy.
Initially starting with simple analgesics (such as paracetamol or NSAIDs), if the pain is not well controlled, move up to the next stage of the ladder and consider prescribing weak opiates, such as codeine. Again, assess the response and if this is still inadequate, move to the next step and prescribe morphine or other strong opiates.
Consider alternatives to the oral route, such as topical, intravenous, or subcutaneous. If this fails and sinister causes of pain have been ruled out, consider specialist help and/or a patient-controlled analgesia pump. Any neuropathic pain may respond better to alternative analgesics (see appendix), such as amitriptyline or gabapentin.
As patients recover, it is important to move down the ladder, and wean down the analgesia to a more simple regime. It is always preferable to not send patients home with strong opiates.
Types of Analgesia
Simple Analgesics
Non-opioid analgesia consists of paracetamol and/or NSAIDs (e.g ibuprofen or diclofenac).
NSAIDs work by inhibiting the synthesis of prostaglandins, thereby reducing the potential inflammatory response causing the pain. These anti-inflammatory properties mean such analgesics are often used in musculoskeletal conditions. They are also frequently used intra-operatively.
The side effects of NSAIDs include (a useful mnemonic is I-GRAB):
- Interactions with other medications (such as Warfarin)
- Gastric ulceration (consider adding a PPI when prescribing NSAIDs long-term)
- Renal impairment (use NSAIDs sparingly in those with poor renal function)
- Asthma sensitivity (triggers 10% of individuals with asthma)
- Bleeding risk (due to their effect on platelet function)
Opiate Analgesics
Opiates are divided into weak opiates, such as codeine, or strong opiates, such as morphine, oxycodone, or fentanyl. They work by activating opioid receptors (MOP, DOP, and KOP), which are distributed throughout the central nervous system.
These medications have a significant side effect profile; most patients will experience a degree of constipation and nausea. Thus, laxatives and anti-emetics should be prescribed concurrently. Other side effects include sedation or confusion, respiratory depression, pruritus, and tolerance and dependence.
Prescribing Tips
- If regular opiates are needed, always prescribe concurrent regular paracetamol to reduce their requirement
- Avoid weak and strong opiates in combination, as they competitively inhibit the same receptor to varying degrees
- If PRN (‘as needed’) opiates are frequently called for, assess the 24-hour opiate requirement and consider titration into a regular basal dose of modified-release preparations.
- If opioid analgesia is required in a patient with renal impairment, consider using oxycodone or fentanyl rather than morphine
- If the oral route is contraindicated, consider topical patches and use IV morphine for breakthrough analgesia, as the bioavailability of oral morphine is 30% whereas it is 80% for IV or SC morphine
- Remember, morphine takes approximately 2-3 minutes to work if given intravenously, 20 minutes if taken orally, and 15 minutes if given intramuscularly
Local Anaesthesia
Local anaesthesia can be used as an adjunct following surgery to optimise pain relief. Typically, local anaesthesia is given intra-operatively, in varying forms, to aid post-operative recovery.
Examples include regional anaesthetic blocks (e.g. serratus anterior block for rib fractures), rectus sheath catheters (infusing local anaesthetic into the posterior rectus sheath), or spinal or epidural anaesthesia (Fig. 2)
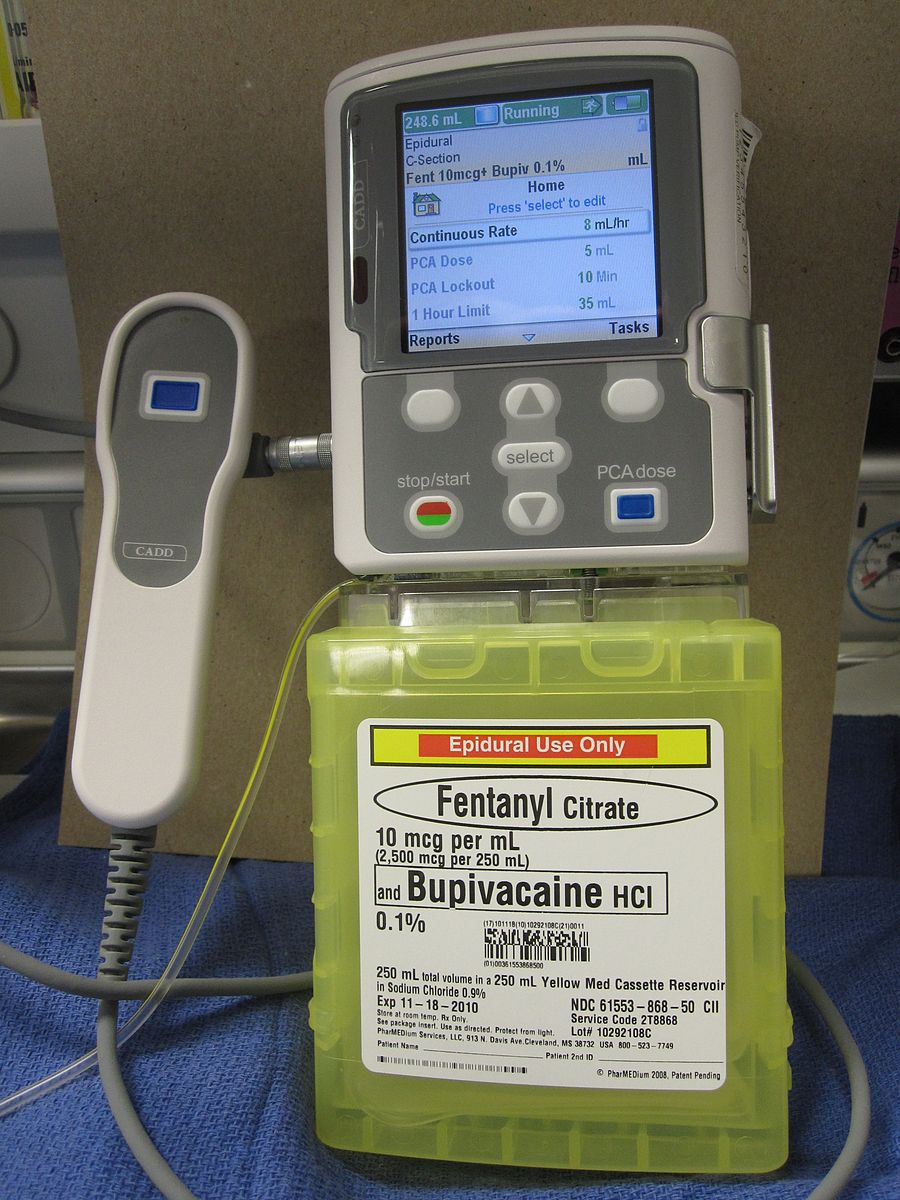
Figure 2 – A PCA pump configured for epidural administration of Fentanyl and Bupivacaine
Patient Controlled Analgesia
Post-operatively, many patients require more intense or immediate analgesia and their requirements exceed the capacity of nursing staff to provide. In such situations, patient controlled analgesia (PCA) can be used (Fig. 2).
PCA involves the use of intravenous pumps that provide a bolus dose of an analgesic when the patient presses a button. These are either started in theatre or on the wards, when the use of strong oral opiates is inadequate.
They can be titrated to give background infusions of analgesia if needed and the analgesic agent used can also vary (e.g. opiates, local anaesthesia).
| Advantages | Disadvantages |
|
|
Key Points
- Pain control is an important competency to develop as a junior doctor and a simple stepwise approach should always be used
- Accurate pain assessment is crucial and will ensure appropriate and safe decision-making when prescribing analgesia
- A basic understanding of the pharmacokinetics of opiates will aid in their prescription
Appendix – Neuropathic pain
Neuropathic pain results from irritation or injury directly to the nerves, either peripherally or centrally. It often presents with shooting or stabbing pains, and can be described as like an electrical shock.
Following surgery, the prevalence of neuropathic pain is as high as 10%. It is frequently encountered in orthopaedic or vascular surgery, such as following limb amputation (due to the nerve damage sustained when the limb is severed).
The management of neuropathic pain can be split into pharmacological and non-pharmacological methods. In many cases a combination of both approaches offers the best results:
- Non-pharmacological treatment – cognitive behaviour therapy, transcutaneous electric nerve stimulation (TENS), or capsaicin cream (typically for localised pain)
- Pharmacological therapies – gabapentin, amitriptyline, or pregabalin; if these are not successful or not tolerated, specialist referral should be considered